FDA Approves AI Algorithms for Detecting Heart Murmurs and Atrial Fibrillation (AFib)
Most people like
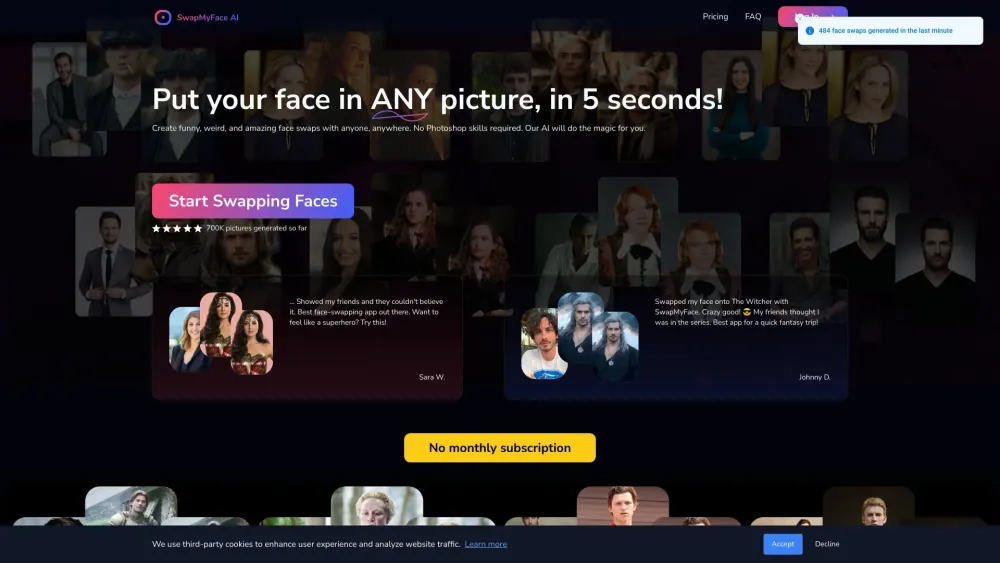
Discover the ultimate AI face swap tool designed for transforming any image effortlessly. This innovative technology allows users to swap faces with ease, resulting in realistic and seamless edits. Whether you're creating fun memes, enhancing your social media posts, or experimenting with creative projects, our user-friendly tool simplifies the process and delivers impressive results. Dive in and experience the magic of AI face swapping today!

Discover top-performing dropshipping products effortlessly with WinningHunter's premier ad spy tool. Analyze Facebook and TikTok advertisements, monitor store sales, and create compelling ad copy using AI technology. Elevate your dropshipping success with WinningHunter’s robust set of features designed for optimal results.
ScriptMe offers rapid and precise transcription and subtitling services in various languages, ensuring high-quality results tailored to your needs.
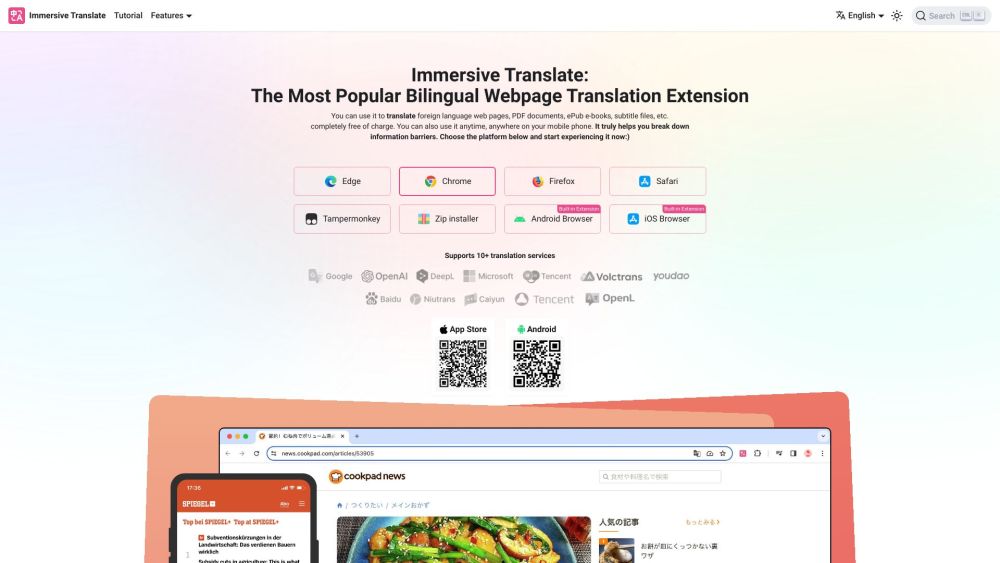
Introducing our free bilingual webpage and document translation tool, designed to make translations effortless and accessible. Whether you need to convert website content or important documents, our intuitive platform ensures clear communication in multiple languages, empowering you to connect with a global audience seamlessly. Translate with ease and enhance your online presence today!
Find AI tools in YBX

